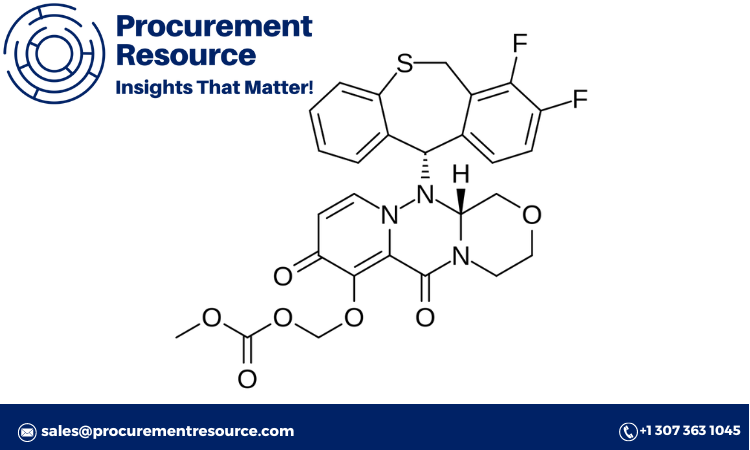
Boloxavir marboxil is a revolutionary antiviral drug used to treat influenza. It works by inhibiting the replication of the influenza virus, offering a valuable alternative to traditional treatments like oseltamivir (Tamiflu). As pharmaceutical companies continue to explore novel therapies for viral infections, understanding the Boloxavir Marboxil Production Cost is crucial for stakeholders, including pharmaceutical manufacturers, investors, and healthcare providers. This comprehensive article will provide a detailed look at the production cost of boloxavir marboxil, covering key components such as the cost model, pre-feasibility studies, labor charges, utilities, logistics, and supply chain factors.
Request a Free Sample for Boloxavir Marboxil Production Cost Reports – https://www.procurementresource.com/production-cost-report-store/boloxavir-marboxil/request-sample
Understanding Boloxavir Marboxil Production Costs
The Boloxavir Marboxil Production Cost refers to the total expenses involved in the synthesis and manufacturing of boloxavir marboxil, including raw materials, labor, energy consumption, facility operations, and logistics. Due to the complexity of the drug’s manufacturing process, the cost structure can be considerably higher than that of traditional drugs, requiring advanced chemical processes and stringent quality control measures.
For pharmaceutical companies, controlling production costs is essential for maintaining profitability, ensuring competitive pricing, and managing market supply. As demand for antivirals like boloxavir marboxil increases, understanding cost drivers will allow companies to optimize production and improve operational efficiency.
Key Factors Affecting Boloxavir Marboxil Production Costs
The production costs of boloxavir marboxil are influenced by a variety of factors, ranging from the raw materials used in synthesis to labor and operational expenses. Below, we explore these key factors in detail.
1. Raw Material Costs
Boloxavir marboxil is synthesized using specialized chemical compounds, some of which are derived from high-quality reagents and intermediates. The cost of these raw materials forms a significant part of the overall production cost. Pharmaceutical-grade chemicals required for boloxavir marboxil synthesis are expensive due to their high purity requirements and complex production methods.
- Sourcing and Procurement: The availability and sourcing of raw materials can impact their cost. Global supply chain disruptions, such as those caused by geopolitical instability or natural disasters, can lead to fluctuations in raw material prices.
- Chemical Intermediates: The production of boloxavir marboxil involves multiple chemical steps, with intermediate compounds being produced at each stage. Any price increases or supply shortages in these intermediate chemicals can directly impact production costs.
In recent years, many pharmaceutical companies have focused on reducing the dependency on scarce raw materials by developing more cost-effective synthetic routes or seeking alternative sources for these intermediates.
2. Labor Charges
Labor costs make up a significant portion of any pharmaceutical production facility’s budget. Skilled personnel are required at every stage of the manufacturing process, from research and development (R&D) to quality control and regulatory compliance.
- Skilled Workforce: The production of boloxavir marboxil requires highly trained chemists, pharmacologists, and engineers. These specialists must ensure that the production process meets stringent regulatory standards, including those set by agencies like the U.S. FDA or the European Medicines Agency (EMA).
- Manufacturing Labor: The manufacturing of boloxavir marboxil involves complex chemical processes that require specialized operators for reactors, distillation units, and filtration systems. Labor charges for operators and technicians are influenced by regional wage rates and the level of automation in the production facility.
Read the Full Report – https://www.procurementresource.com/production-cost-report-store/boloxavir-marboxil
While automation can reduce labor costs in large-scale manufacturing, the need for skilled human oversight remains, particularly in the case of high-value medications like boloxavir marboxil.
3. Utilities and Energy Consumption
Pharmaceutical production is energy-intensive, and the manufacturing of boloxavir marboxil is no exception. Utilities, such as electricity, water, and gas, are required for various processes, including heating, cooling, filtration, and sterilization.
- Electricity and Gas: The chemical reactions involved in boloxavir marboxil production often require high temperatures, making natural gas or electricity a significant utility expense. The cost of energy can fluctuate based on regional pricing, with some countries having higher utility costs than others.
- Water Usage: Water is crucial in the production of boloxavir marboxil, both for cooling purposes and as a solvent in some stages of the chemical process. In regions with limited water resources, costs can be higher due to water treatment and procurement costs.
Energy costs, in particular, have been a key focus for pharmaceutical companies as they look for ways to optimize consumption and reduce operational expenses. Some companies have adopted green energy sources or energy-efficient technologies to mitigate rising utility costs.
4. Operational Costs
Operational costs in boloxavir marboxil production include expenses related to manufacturing facilities, equipment, and quality control. Pharmaceutical production facilities must adhere to strict guidelines for cleanliness, contamination control, and safety, which adds to the operational cost.
- Facility Maintenance and Depreciation: The capital cost of building and maintaining a production facility is high. Manufacturing plants must be regularly maintained to ensure smooth operations. Depreciation of equipment and infrastructure is an ongoing expense that must be accounted for.
- Quality Control and Testing: Since boloxavir marboxil is a drug that must meet high purity and safety standards, extensive quality control measures are required at various stages of production. This includes testing raw materials, intermediate products, and the final formulation. The cost of quality control staff and equipment is considerable.
Ask an Analyst – https://www.procurementresource.com/production-cost-report-store/boloxavir-marboxil/ask-an-analyst
5. Logistics and Supply Chain Costs
The logistics of getting raw materials to the production site and delivering finished products to market can significantly affect the overall cost of boloxavir marboxil production. As with any pharmaceutical product, supply chain management is crucial to ensuring that materials are available when needed and that the finished product reaches healthcare providers in a timely manner.
- Raw Material Transportation: The transportation of raw materials to the production facility incurs costs related to fuel, freight, and warehousing. In some cases, bulk raw materials need to be sourced internationally, adding complexity to logistics.
- Distribution of Finished Product: Once boloxavir marboxil is produced, it needs to be distributed to pharmacies, hospitals, and other healthcare providers. Transportation costs for the final product include both domestic and international shipping, as well as distribution fees.
- Supply Chain Optimization: Efficient supply chain management can help reduce costs. Many pharmaceutical companies are adopting just-in-time (JIT) inventory systems to minimize storage costs and ensure that raw materials and finished products are delivered exactly when needed.
Pre-Feasibility Studies for Boloxavir Marboxil Production
Before embarking on large-scale production of boloxavir marboxil, a pre-feasibility study is crucial to evaluate the economic viability of the project. This study typically includes an analysis of the expected production costs, potential market demand, competitive landscape, and regulatory hurdles. Key components of a pre-feasibility study include:
- Market Analysis: Estimating demand for boloxavir marboxil is vital for understanding whether the production costs can be recouped through sales. This includes analyzing current and future trends in influenza treatment and antiviral drug markets.
- Cost Modeling: Detailed cost models, including capital investment, operational costs, and expected profit margins, are developed to assess whether production is financially viable.
- Risk Assessment: A thorough risk assessment helps identify potential challenges, such as fluctuations in raw material prices, regulatory issues, or changes in market demand.
- Regulatory Considerations: Pharmaceutical production involves strict regulatory oversight, and understanding these requirements is key to ensuring that the project remains on track.
Request Your Free Sample – https://www.procurementresource.com/production-cost-report-store/boloxavir-marboxil/request-sample
Industrial Trends in Boloxavir Marboxil Production
The production of boloxavir marboxil, like other pharmaceuticals, is subject to a range of industrial trends, including advancements in manufacturing technology, increased focus on sustainability, and shifts in global healthcare policies. Some notable trends include:
- Green Chemistry: The rise of green chemistry practices has led pharmaceutical manufacturers to adopt more sustainable production methods. These methods focus on reducing waste, energy consumption, and the use of toxic chemicals, which can lower the overall cost of production in the long term.
- Outsourcing and Contract Manufacturing: Many pharmaceutical companies outsource the production of certain intermediates or even entire products to contract manufacturers. This allows companies to reduce capital investment and focus on other aspects of the business.
Contact Us
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Numbers:
USA : 1 307 363 1045
UK: 44 7537171117
Asia-Pacific (APAC): 91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA




Leave a Reply